Measuring alcohol concentration
This measurement can be made in two ways:
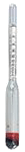 Using an alcoholmeter (densimeter graduated in % vol alcohol and calibrated at 20° C). This method can only be used when the alcohol-water mixture contains no extract (sugar...) or after a quantitative distillation. The measure performed at another temperature than 20° C will be automatically corrected by the software.
Using an alcoholmeter (densimeter graduated in % vol alcohol and calibrated at 20° C). This method can only be used when the alcohol-water mixture contains no extract (sugar...) or after a quantitative distillation. The measure performed at another temperature than 20° C will be automatically corrected by the software.
Warning: the equipment (test-tubes and alcoholmeter) has to be perfectly clean and free from any fat; the measured liquid must not be sparkling.
The alcohol-water mixtures containing dry extract (sugar) have to be measured either after a quantitative distillation (that necessitates a know-how and a special material), or measured by difference: the mixture density is measured (d1), the alcohol is eliminated by evaporating a sample (100 mL) of the liquid to half its volume, and bringing the volume to the initial volume with pure water, then a second measure of density is made (d2). The alcohol concentration is calculated by the following formula: 1-d2+d1.
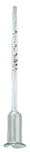 By capillarity, using a vinometer, small device calibrated from 0 to 15 or 25 % vol. It is filled with a few drops of the liquid to be measured. It is put on a dry surface; the top of the liquid column will slope down and stabilize after 2-3 minutes at a gradation level corresponding with the alcoholic percentage. It can be necessary, prior to measuring, to dilute the liquid if the alcohol concentration is high (the measure is the most accurate under 10 % vol).
By capillarity, using a vinometer, small device calibrated from 0 to 15 or 25 % vol. It is filled with a few drops of the liquid to be measured. It is put on a dry surface; the top of the liquid column will slope down and stabilize after 2-3 minutes at a gradation level corresponding with the alcoholic percentage. It can be necessary, prior to measuring, to dilute the liquid if the alcohol concentration is high (the measure is the most accurate under 10 % vol).
Caution: the vinometer must be very clean and defatted (pure alcohol) after each use. The fizzy liquids must be degassed (by agitation, filtration, heating...), the liquid column must be continuous (no bubbles). In the case of sugar containing liquids (sweet wine, density>0.998), the vinometer gives a somewhat too high measure.